Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review
Abstract
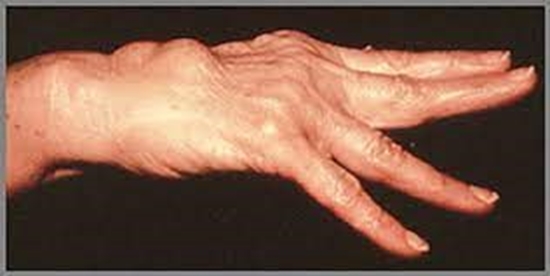
Three important factors, including genetics, environment factors and autoimmunity play a role in the pathogenesis of rheumatoid arthritis (RA). The heritability of RA has been accounted to be 50–60%, while the HLA involvement in heritability of the disease has been accounted to be 10–40%. It has been documented that shared epitope (SE) alleles, such as HLA-DRB1*01 and DRB1*04, some HLA alleles like HLA-DRB1*13 and DRB1*15 are connected to RA susceptibility. An advanced classification of SE categorizes SE alleles into four main groups namely, S1, S2, S3D, and S3P. The S2 and S3P groups have been linked to susceptibility of seropositive RA. Various genome-wide association studies (GWAS) have discovered many susceptibility loci implicated in pathogenesis of RA. Some of the important single nucleotide polymorphisms (SNPs) linked to RA are TRAF1, STAT4, CTLA4, IRF5, CCR6, PTPN22, IL23R, and PADI4. HLA and non-HLA genes may discriminate anti-cyclic citrullinated peptide (anti-CCP) antibody–positive and anti–CCP–negative RA groups. Furthermore, risk of the disease has also been linked to environmental agents, mainly cigarette smoking. Pharmacogenomics has also confirmed SNPs or genetic patterns that might be linked to drugs responses. Different aspects of genetic involvement in the pathogenesis, etiology, and RA complications are reviewed in this article. © 2019 Elsevier B.V.
Author keywords
Genetics; HLA shared epitope; Pathogenesis; Rheumatoid arthritis







ارسال به دوستان